Background
After years of researching water's impact on coffee attributes, we concluded in late 2021 that this effect stems from individual minerals in water — specifically, the role of each ion present.
This approach was, and largely still is today, a revolutionary way of addressing this fascinating and complex topic. Early coffee literature viewed water in a simpler, binary manner. It often considered only a few select ions (mainly Magnesium, Calcium, Carbonate, and Bicarbonate) and categorised them into two groups: General Hardness (Magnesium + Calcium) and Alkalinity (Carbonate + Bicarbonate). We found several flaws in this simplistic approach, the main one being that it overlooked many other ions present in water.
In a liquid medium, ions can move freely and individually. However, in solid form, they must attach to other ions with an opposite electrical charge (positives bind with negatives and vice versa). This means you can't simply add solid Magnesium or Calcium to water on their own—they must be paired with other ions such as Chloride, Sulfate, or Carbonate.
Many of these ions that don't fit into the GH/KH categories are often dismissed and overlooked. We believe that every single ion present in water plays a key role in influencing the flavours in your final cup of coffee. We've spent years developing a better understanding of how these individual ions affect coffee.
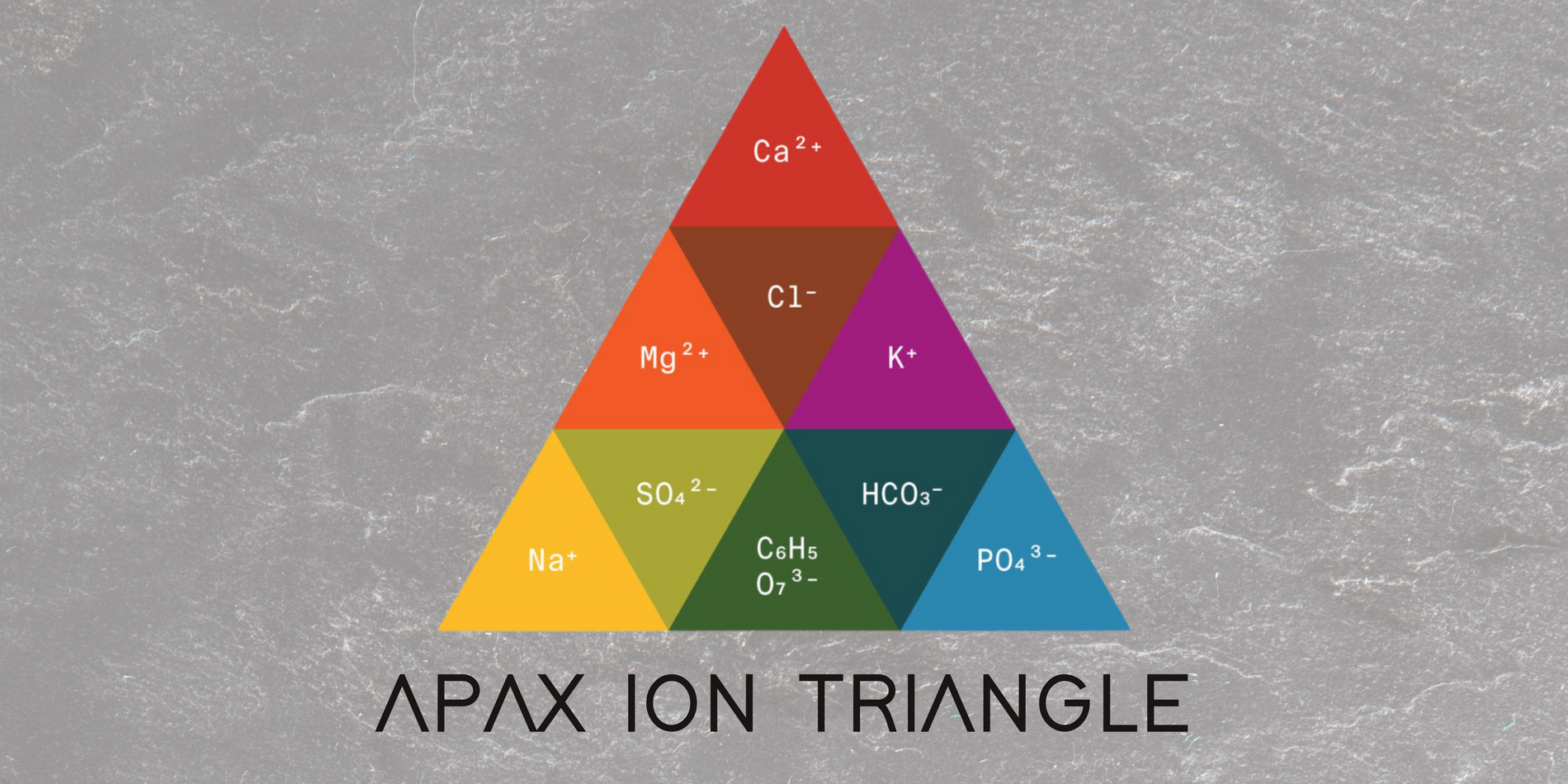
What is the APAX Ion Triangle and how to interpret it
Introducing the APAX ION Triangle — a framework we created to gather nine of the most powerful and commonly found ions in water in the context of coffee brewing.
By collecting extensive data from tasting panels conducted by industry experts, we've crafted an accurate profile for each of these ions and their role in coffee.
Each ion can bring positive attributes to coffee when used in the correct amounts. However, excessive concentrations lead to negative impacts. It's similar to adding salt and lemon to your food: the first pinch or squeeze is generally beneficial, but as you add more, the benefits diminish until adding too much yields negative results.
We focused on understanding the positives and negatives each ion can bring to coffee. This information is particularly useful when you're trying to improve coffee. If you know which ion can make coffee sweeter or heavier, you know precisely what you should add.
Conversely, if your coffee shows signs of dryness and lacks acidity because you've pushed the limits of ion concentration, you'll know which ones to decrease to fix this issue.
Precise knowledge about each ion's impact on coffee flavours becomes a new tool in your arsenal—and likely the most powerful and precise one.
The Individual Impact of Ions

Calcium is a positively charged ion (cation) that is ubiquitous in water, being one of the most prevalent and influential minerals found in natural water sources. Its presence plays a crucial role in shaping the sensory profile of coffee. When used judiciously, calcium proves to be an exceptionally potent and valuable tool for enhancing the overall coffee experience. It has the remarkable ability to impart a fuller body to the brew, creating a more substantial mouthfeel that many coffee enthusiasts appreciate. Additionally, calcium significantly contributes to the perceived sweetness of the coffee, often resulting in a more rounded and pleasant flavour profile.
One of calcium's most intriguing effects is its tendency to accentuate specific fruit notes in coffee. It has a particular affinity for highlighting red, pink, and purple fruit flavours, which can add depth and complexity to the cup. These fruit notes might include hints of strawberry, raspberry, cherry, or even more exotic fruits like pomegranate or plum, depending on the coffee's origin, processing, variety and roast profile.
However, it's crucial to exercise caution when working with calcium, as its effects require a delicate balance. While moderate amounts can enhance the coffee's qualities, excessive concentrations of calcium can swiftly lead to undesirable outcomes. An overabundance of calcium can result in a dry, chalky texture that detracts from the coffee's natural smoothness. This excessive dryness can be particularly noticeable in the aftertaste, leaving an unpleasant sensation on the palate. Furthermore, too much calcium can have a muting effect on the coffee's overall flavour profile, subduing the nuanced notes and aromatics that make each coffee unique. This can result in a flatter, less vibrant cup that fails to showcase the coffee's full potential.

Magnesium is another prevalent cation found in water, playing a significant role in shaping coffee's flavour profile. It acts as a potent flavour enhancer, contributing to a brighter and more vibrant cup of coffee. Magnesium's influence is particularly noticeable in its ability to accentuate fruity notes, often bringing out delightful hints of citrus, tropical fruits, and stone fruits. These fruit notes can range from orange and lime to exotic mango and papaya, or even subtle peach and apricot undertones.
The presence of magnesium tends to elevate the overall complexity of the coffee, making it more dynamic and interesting on the palate. It can enhance the perceived acidity in a positive way, creating a lively and refreshing sensation that many coffee enthusiasts appreciate. This brightness often translates to a cleaner, crisper finish, leaving a pleasant aftertaste that invites another sip.
However, as with many elements in coffee brewing, balance is key. While moderate amounts of magnesium can significantly improve the coffee experience, excessive concentrations can lead to less desirable outcomes. An overabundance of magnesium may introduce unwanted bitterness, potentially overwhelming the coffee's natural flavours. It can also impart a salty taste, which, while interesting in small amounts, can become overpowering and unpleasant when too pronounced. Additionally, high levels of magnesium can contribute to an increased sense of dryness in the mouth, potentially detracting from the coffee's smoothness and overall enjoyment.

Chloride is a negatively charged ion (anion) that is not commonly found in water used for coffee brewing. Its presence is often avoided due to its potential harmful effects on coffee machine boilers, where it can cause various types of corrosion. As a result, water treatment processes for espresso machines typically involve removing or filtering out chlorides to protect the equipment's longevity and performance.
Despite its less-than-stellar reputation in the context of espresso machine maintenance, chloride has a surprising and significant impact on coffee flavour. In our extensive research and tasting experiments, we found chloride to be one of the most intriguing and potentially beneficial ions for enhancing coffee's sensory profile. When used judiciously, it demonstrates a remarkable ability to elevate key aspects of the coffee experience.
Chloride's primary contributions to coffee flavour include: Enhanced Sweetness: It can significantly boost the perceived sweetness in the cup, adding a pleasant, rounded quality to the overall flavour profile. Improved Roundness: Chloride helps to smooth out any harsh edges in the coffee's taste, contributing to a more balanced and harmonious flavour experience. Increased Intensity: It has the ability to amplify the coffee's inherent flavours, resulting in a more robust and full-bodied cup.
However, as with many elements in coffee brewing, the key lies in finding the right balance. While moderate amounts of chloride can dramatically improve the coffee experience, excessive concentrations can lead to less desirable outcomes. When present in high levels, chloride can introduce an overly salty taste that can overpower the coffee's natural flavours. It may also impart a distinct metallic undertone that can detract from the coffee's intended flavour profile.
These potential drawbacks underscore the importance of precise control and careful consideration when incorporating chloride into water for coffee brewing. The goal is to harness its positive attributes while avoiding the negative impacts that can arise from overuse.

Potassium is a cation that, while less commonly utilised in coffee preparation, plays a significant role when present. It is most frequently encountered in the form of potassium bicarbonate, where it is primarily employed for its bicarbonate ion. Our extensive research suggests that potassium contributes substantially to enhancing the coffee's flavour profile. It has the remarkable ability to elevate both sweetness and body in the cup, doing so in a more nuanced and gentle manner compared to calcium.
Although potassium's impact has a lower threshold than calcium, its effects are nonetheless profound. It possesses the capacity to dramatically improve the coffee's aftertaste, creating a lingering and pleasant finish that persists on the palate. Notably, it achieves this without introducing the chalky texture that can sometimes occur with excessive calcium use. This makes potassium an excellent choice for fine-tuning the sensory experience of coffee, particularly in situations where a subtle enhancement is desired.
However, as with all elements in coffee brewing, balance is key. While moderate levels of potassium can significantly elevate the coffee experience, excessive concentrations can lead to less desirable outcomes. High levels of potassium have been observed to introduce unwanted bitterness to the brew. Additionally, an overabundance of this ion can impart a distinct metallic taste, potentially overshadowing the coffee's natural flavours. Therefore, careful consideration and precise measurement are crucial when incorporating potassium into water for coffee brewing, ensuring that its beneficial properties are harnessed without tipping into negative territory.

Sodium, our final cation in this exploration, is an extremely prevalent ion whose role in coffee brewing is often misunderstood. The most common form of sodium encountered in everyday life is sodium chloride, better known as table salt. In the culinary world, salt is ubiquitous and primarily utilised to enhance and intensify flavours. When it comes to coffee, sodium plays a similarly nuanced role. It has the remarkable ability to accentuate sweetness, heighten brightness, and add vibrancy to the cup. Additionally, sodium can help mitigate perceived bitterness, resulting in a more balanced flavour profile.
One of sodium's most intriguing properties is its capacity to act as a flavour amplifier. Much like in cooking, where a pinch of salt can bring out the inherent flavours of a dish, small amounts of sodium in coffee can elevate and clarify the coffee's natural characteristics. This enhancement effect can make fruity notes more pronounced, floral aromas more distinct, and overall complexity more apparent.
However, it's crucial to exercise caution when incorporating sodium into coffee brewing. While moderate levels of sodium can significantly improve the sensory experience, excessive concentrations can lead to less desirable outcomes. High levels of sodium can impart an overtly salty taste to the coffee, potentially overwhelming its natural flavours. Furthermore, excessive sodium can introduce an unwanted savoury quality, shifting the coffee's profile away from its intended taste characteristics.
The relationship between sodium and coffee is a delicate dance of enhancement and potential detraction. When used judiciously, sodium can be a powerful tool in crafting a more vibrant, sweet, and harmonious cup of coffee.

Sulfate, an anion widely utilised in the brewing industry to enhance hop characteristics, also plays a crucial role in coffee flavour development. This versatile ion demonstrates a remarkable ability to intensify and accentuate floral and citrus notes in coffee, adding depth and complexity to the cup. Additionally, sulfate contributes a subtle yet well-balanced bitterness that can enhance the overall flavour profile when present in appropriate quantities.
The impact of sulfate on coffee is multifaceted. It can brighten the cup by amplifying the perceived acidity, particularly in coffees with inherent citrus or floral qualities. This enhancement can result in a more vibrant and lively taste experience, with heightened aromatics and a cleaner finish. The gentle bitterness imparted by sulfate can also help to round out the flavour, providing a counterpoint to sweetness and acidity that adds dimension to the overall flavour profile.
However, it's crucial to note that the benefits of sulfate in coffee are highly dependent on concentration. While moderate levels can significantly improve the sensory experience, excessive amounts can lead to undesirable outcomes. High concentrations of sulfate can overwhelm the delicate balance of flavours, potentially rendering the coffee harsh, astringent, and excessively bitter. This can mask the coffee's natural nuances and result in a less enjoyable drinking experience.
Careful calibration of sulfate levels is therefore essential when crafting water profiles for coffee brewing. The goal is to harness sulfate's ability to enhance floral and citrus notes and contribute a balanced bitterness, while avoiding the pitfalls of overuse.

The citrate ion comes from citric acid, which is the acid found in citrus fruits like lemons and oranges. Citric acid gives these fruits their sour taste and can also enhance citrus-like qualities in coffee.
When citric acid dissolves in water, it dissociates into hydrogen ions (which contribute to sourness) and citrate ions. The citrate ion itself doesn't have a sour taste, but it plays a role in the overall acidity of the solution. The sourness you perceive from citric acid is primarily due to the presence of the hydrogen ions, not the citrate ion directly.
In simple terms, while citrate is involved in the sour taste by being part of the citric acid structure, the actual acidity and sourness mainly come from the hydrogen ions released by citric acid.

Bicarbonate is an ion commonly found in water and is renowned for its buffering capabilities. This means it plays a crucial role in maintaining the pH balance of water by neutralising acids. Through this buffering action, bicarbonate effectively reduces the acidity of water, resulting in an increase in its pH level. This property makes bicarbonate a significant factor in water chemistry, particularly when it comes to its effects on coffee brewing.
In the context of coffee, bicarbonate serves multiple functions that significantly impact the final cup. Primarily, it acts as a balancing agent, working to reduce and moderate the acidity in coffee. This reduction in acidity contributes to a smoother, more rounded flavour profile, often described as having increased body and improved structure. The presence of bicarbonate can help to soften harsh or overly bright notes, creating a more harmonious taste experience. Additionally, it can enhance the perception of sweetness in the cup, making it particularly beneficial for certain coffee varieties or roast profiles.
However, as with many elements in coffee brewing, the key lies in achieving the right balance. While moderate levels of bicarbonate can significantly improve the coffee experience, excessive concentrations can lead to less desirable outcomes. An overabundance of bicarbonate may result in over-neutralisation of the coffee's natural acidity. This can lead to a flat or dull taste profile, where the vibrant, nuanced flavours that make each coffee unique are muted or lost entirely. In extreme cases, high levels of bicarbonate can even impart an unpleasant, alkaline taste to the coffee, detracting from its overall quality and enjoyment.

Phosphate is an anion that plays a crucial role in balancing acidity in coffee without completely neutralising it, as bicarbonate would. This unique property allows phosphate to maintain a delicate equilibrium in the coffee's flavour profile. When used in the right amounts, phosphate can enhance the clarity and vibrancy of the brew, bringing out subtle nuances and brightening the overall taste experience. It acts as a flavour enhancer, helping to articulate the coffee's inherent characteristics more distinctly.
However, as with many elements in coffee brewing, the key lies in achieving the right balance. While moderate levels of phosphate can significantly improve the coffee experience, excessive concentrations can lead to less desirable outcomes. An overabundance of phosphate may result in a flattening effect on the coffee's flavour profile, muting its complexity and depth. Additionally, high levels of phosphate can impart an unwanted dryness to the brew, potentially detracting from its smoothness and overall enjoyment. This drying effect can manifest as an astringent sensation on the palate, altering the coffee's mouthfeel and finish.
Therefore, when incorporating phosphate into water for coffee brewing, precision and careful consideration are crucial. The goal is to harness its positive attributes – the ability to balance acidity and enhance clarity – while avoiding the negative impacts that can arise from overuse.
Methodology
To better understand the individual impact of specific ions on coffee, we conducted extensive triangulation tests, cross-tastings, and blind sensory sessions with licensed Q Graders, World Champions, and other coffee and water experts.
To isolate ions and assess their effects, we performed multiple cross-referencing tests by tasting the same coffee with different mineral additions, such as Magnesium Chloride, Magnesium Sulfate, Magnesium Carbonate, as well as Sodium Chloride, Sodium Bicarbonate, and Sodium Phosphate.
By tasting the same coffee with only one type of mineral (or ion pair) added, we were able to discern the specific contributions of each ion. For instance, we consistently found that all three magnesium compounds tested—Magnesium Chloride, Magnesium Sulfate, and Magnesium Carbonate—enhanced fruitiness, sweetness, and brightness in the coffee. This suggests that the magnesium ion itself is responsible for these attributes, as it is the common element among the three. Differences in the resulting flavour profiles could then be attributed to the accompanying ions (chloride, sulfate, and carbonate), providing us with a precise understanding of each ion's individual impact.
These tests were conducted across a variety of coffees, including different roast profiles, origins, processing methods, and brewing techniques, and involved dozens of different minerals.
We were ultimately satisfied with our findings when we could consistently identify which minerals had been added to the coffee during blind tastings, determine the concentration levels, and accurately predict the flavour outcomes when introducing a specific mineral to a coffee.
To replicate these tests yourself, brew a large batch of coffee, preferably using a batch brewer. Divide the coffee into eight cups and add between 60 and 120 ppm of eight different minerals, one mineral per cup. Discreetly mark the bottom of each cup, shuffle them, and conduct a blind tasting. Challenge yourself to guess which mineral was added to each cup.
This article was written by Simon Gautherin, Q Arabica Grader, Head of Training and Education at Zest Coffee, and designer of the APAX LAB mineral profiles. I would like to extend my heartfelt thanks to those who contributed their expertise and insights to this work: Amy Chang, water sommelier based in Taiwan and fellow Q Arabica Grader; Young Baek, World Cup Tasters Champion 2023; Carlos Escobar, Brewers Cup Champion (4th place) 2021; Jamie Thomson, Head of Coffee for Golden Brown and passionate water enthusiast; and Monika Fekete, chemical scientist and water expert; and Amy Ruse, who designed and brought to life the graphs and frameworks used in this article. Your valuable contributions have greatly enriched this article, and for that, I am deeply grateful.
Click here to download the APAX ION TRIANGLE.






2 comments
Hi Denis, 120mg/l sulfates is likely too much.
A common recipe for filter coffee is 145mg/l hydrous Magnesium Sulfate and 5mg/l Calcium Carbonate, for about 56mg/l sulfates.
Mineralized espresso water would typically have fewer sulfates.
Hello, fascinating article! Do you have specific ranges (in mg/l) to aim for with these ions? I am specifically interested in sulfates because I can’t find any reliable information on them. Is around 120mg/l OK or too much?